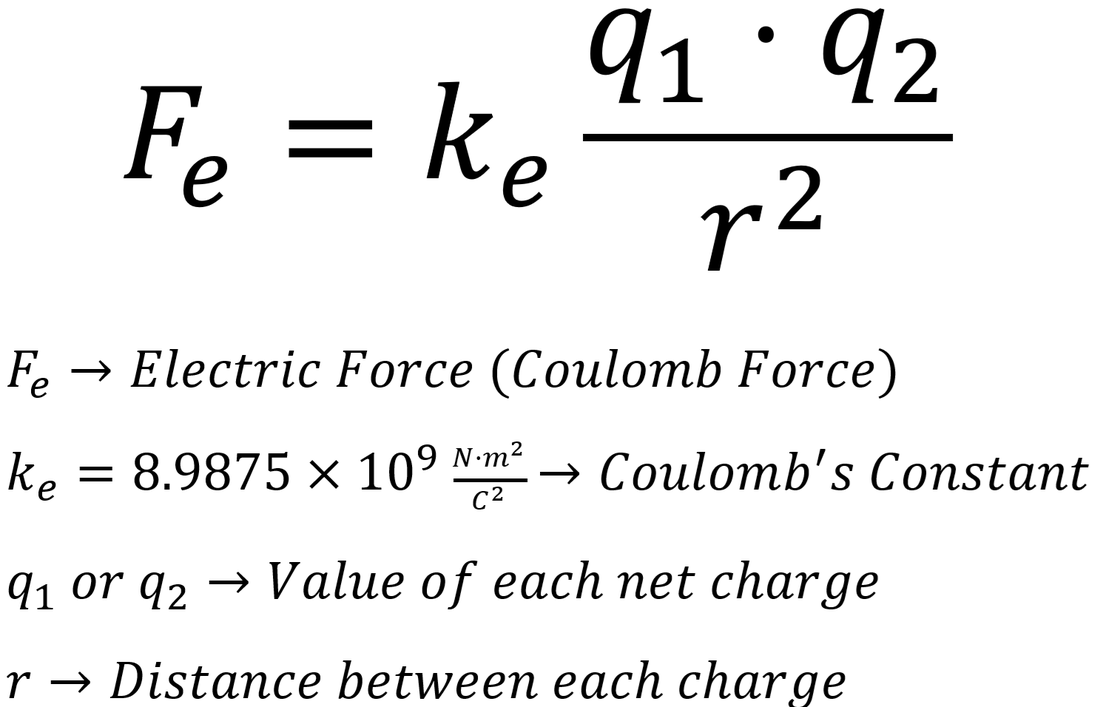What is Electric Charge?
|
What is electric charge? This is a very good question, and one that might be harder to answer than you think. For our purposes, we will say that electric charge is a conserved physical property of matter that causes it to experience a force when placed in an electromagnetic field. This definition requires us to also understand electromagnetic fields. You will probably have to do your best to take on faith there exists a physical property called electric charge, and we can measure it and use it to make predictions about the world around us. There are two types of electric charges; POSITIVE and NEGATIVE. If an object has an equal number of positive and negative charges, we call that object NEUTRAL. The variable to represent charge is q, and the unit of charge is a Coulomb.
|
Red and blue are often colors associated with positive and negative charges. However, red does not necessarily mean positive.
|
Electric Force
At this point in your life, you have probably heard the term, "opposites attract." This phrase is largely attributed to positive and negative charges, and electrostatics. This means that positive and negative charges are pulled towards each other, while like charges (positive/positive and negative/negative) will push away from each other. We know that pushes and pulls are actually forces, so we can calculate the force between the interactions. This electric force is more commonly referred as Coulomb's Law:
|
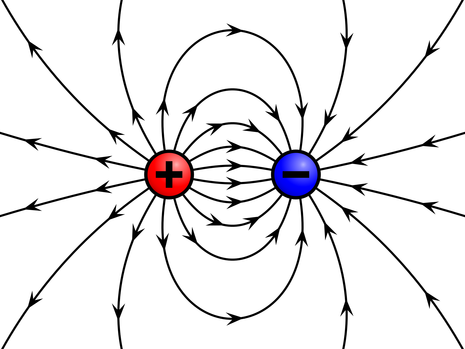
This allows us to calculate the force between two electrically charged objects. A single charged particle, or set of charged particles, creates an electric field around it. The electric field is a region where an electric force would be exerted on other charged particles or objects
Drag and drop a charge into the "Charges and Fields" simulation. Then drag a "sensor" into the simulation. The simulation show the direction and strength of the electric field. The sensor shows the electric force vector that a positive charge would experience within the electric field. Notice the force vector increases as the sensor moves towards each charge, but the direction tries to point away from positive charges, and towards negative charges. If the sensor were a negative charge, the direction of the forces would change, but the magnitude would remain the same. |
|
Conductors & Charged Systems
So how do objects become charged? Where do we get negative and positive charges. For simplicity sake, the source of all of our charges will come from two subatomic particles: electrons and protons. In 1909, Robert Millikan completed his famous Oil Drop Experiment which determined the elementary charge. The elementary charge is the smallest possible charge that an object can have. This means charge is quantized, it cannot be any value, only multiples of the elementary charge. The elementary charge is the value of the charge on a single proton (or the magnitude of the charge of a single electron). This means, for our purposes, all of our net charges will come from either more protons than electrons in a system (positive charge) or more electrons than protons in a system (negative charge).
So how do we get more protons than electrons in a system, or more electrons than protons? IT is important to know hear that it is very hard to move protons around. Protons exist in the nucleus, and if one were to leave the atom, it would become a completely different element! not to mention that a proton flying around would almost certainly attract another electron, making it neutral. However, electrons leave atoms all the time. Valence electrons often move from one atom to another without much difficulty. So if we a have a net charge, that s because electrons are moving, NOT protons.
So how do we get more protons than electrons in a system, or more electrons than protons? IT is important to know hear that it is very hard to move protons around. Protons exist in the nucleus, and if one were to leave the atom, it would become a completely different element! not to mention that a proton flying around would almost certainly attract another electron, making it neutral. However, electrons leave atoms all the time. Valence electrons often move from one atom to another without much difficulty. So if we a have a net charge, that s because electrons are moving, NOT protons.
Materials can be either conductors, or insulators when we talk about transferring charge. Conductors allow charge to flow freely, while insulators allow charges to be rearranged slightly, although they cannot flow. When insulators rub against each other, some electrons can transfer and "stick" to another. Take the balloon above and rub it on the sweater. The balloon is able to gain electrons, giving it a net negative charge. Because charge is conserved, the sweater loses those electrons and it now has a net positive charge. If you let the balloon go, it (being negatively charged) will be pulled towards the positively charged sweater. However, even though the wall is negatively charged, when you bring the negative balloon near by, the electrons are pushed away creating an area of positive charge to which the balloon sticks. So charged objects are attracted to neutral objects if they can polarize the dielectrics. Polarized systems have one area of positive charge and another of negative charge even though the net charge is zero. Feel free to make more changes with the simulations but notice how everything behaves when the net charges are larger.